Details of the Drug
General Information of Drug (ID: DMBGWPH)
| Drug Name |
Medroxyprogesterone
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Asconale; Colirest; Hematrol; Hydroxymethylprogesterone; Lunelle; Medrossiprogesterone; Medroxiprogesterona; Medroxiprogesteronum; Medroxyprogesteron; Medroxyprogesteronum; Methylhydroxyprogesterone; Adgyn Medro; Farlutal inyectable; Medroprogesterone Acetate; Medrossiprogesterone [DCIT]; Medroxyprogesteron acetate; Medroxyprogesterone Strakan Brand; Sodelut G; Strakan Brand of Medroxyprogesterone; MPA Gyn 5; U 8840; CBP-1011; Farlutal inyectable (TN); G-Farlutal; Medroxiprogesterona [INN-Spanish]; Medroxyprogesterone (INN); Medroxyprogesterone [INN:BAN]; Medroxyprogesteronum [INN-Latin]; Novo-Medrone; Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6alpha)-(9CI); Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6-alpha)-(9CI); (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one; (6alpha)-17-hydroxy-6-methylpregn-4-ene-3,20-dione; 17 alpha Hydroxy 6 alpha Methylprogesterone; 17 alpha-Hydroxy-6 alpha-Methylprogesterone; 17-Hydroxy-6.alpha.-methylprogesterone; 17-Hydroxy-6alpha-methyl-pregn-4-ene-3,20-dione; 17-Hydroxy-6alpha-methylprogesterone; 17.alpha.-Hydroxy-6.alpha.-methylprogesterone; 17alpha-Hydroxy-6alpha-methyl-4-pregnene-3,20-dione; 17alpha-Hydroxy-6alpha-methylprogesterone; 6-Dihydromegestrol; 6-alpha-Methyl-17-alpha-hydroxyprogesterone; 6.alpha.-Methyl-17.alpha.-hydroxyprogesterone; 6alpha-Methyl-17alpha-hydroxyprogesterone; 6alpha-Methyl-4-pregnen-17alpha-ol-3,20-dione; 6alpha-Methyl-5-pregnen-17alpha-ol-3,20-dione
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Contraceptive Agents
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
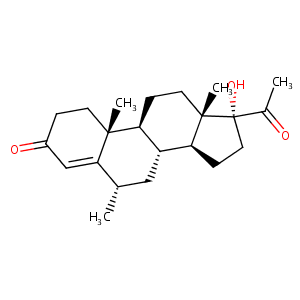 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 344.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
